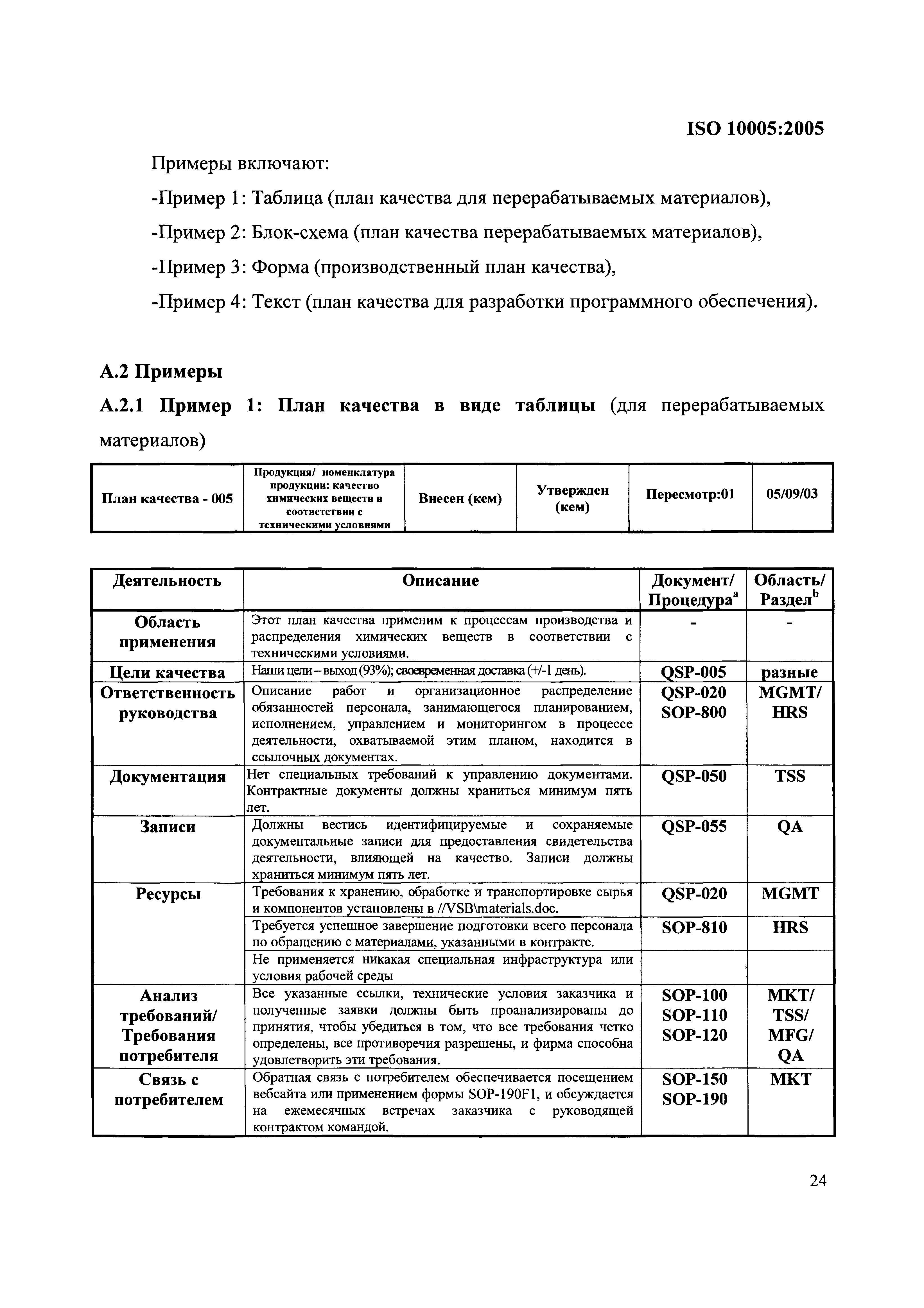
Iso 10005 Quality Plan Template
Iso 10005 Quality Plan Template - The quality plan should include or make reference to the plan(s) for design and development. 4.4 preparation of the quality plan. Web iso 10005 was created in response to the growing demand for quality management systems, and it can be applied to any organization. 4 development of a quality plan. Iso 10005:2005 provides guidelines for the development, review, acceptance, application and revision of quality plans.
In general, quality plan is not a specific requirements from the iso 13485:2016 or from fda quality system regulation 21 cfr 820; It helps in maintaining the improved quality. Applicable where a quality plan is to be used for a particular product, project or contract. It is applicable whether or not the organization has a management system in conformity with iso 9001. The guide has just been updated to provide more guidance and examples to be relevant to organizations of all shapes and sizes. Web iso 10005 was created in response to the growing demand for quality management systems, and it can be applied to any organization. Text in blue indicates what information should be replaced with that text.
Quality Plan Template 000 Risk Management Computing
Web 4 using a quality plan. Web clause 5 describes the process of developing a quality plan. Web the guidance on quality plans in this document is based on the quality management principles described in iso 9000 and the concepts used in iso 9001 for the establishment of quality management systems. The guide has just.
BETTER Iso 10005 Quality Plan Template 🤟🏻 Coub
This document gives guidelines for establishing, reviewing, accepting, applying and revising quality plans. Web project quality plan (pqp) cpd accredited provider. Clause 7 describes the operation and control of a quality plan. Text in blue indicates what information should be replaced with that text. 4.2 inputs to the quality plan. Clause 6 describes the typical.
QMS Quality Management Plan (ISO 10005) Word Template ISO Templates
These guidelines are not intended to be used as a checklist for compliance with requirements. The quality standards are metrics to describe the successful outcome of a project deliverable. These are the sections recommended for a typical quality plan. Clause 6 describes the typical contents of a quality plan. 4.3 scope of the quality plan..
ISO 100052005 Quality management systems — Guidelines for quality plans
Clause 6 describes the typical contents of a quality plan. 4.4 preparation of the quality plan. 4.1 identifying the need for the quality plan. Clause 7 describes the operation and control of a quality plan. However it is an essential tool that can help medical device manufacturer to deliver on specific projects. Critical components of.
Management Template Iso 10005 Quality Plan Template. Iso 10005
Annex a provides examples of simple quality plans. It is applicable whether or not the organization has a management system in conformity with iso 9001. The quality plan should take account of applicable codes, standards, specifications, quality characteristics and regulatory requirements, as appropriate. Iso 10005:2005 is applicable to quality plans for a process, product, project.
ISO 100052018 Quality management — Guidelines for quality plans
The quality standards are metrics to describe the successful outcome of a project deliverable. 4 development of a quality plan. 4.3 scope of the quality plan. These guidelines are not intended to be used as a checklist for compliance with requirements. 5 content of the quality plan. When complete, this document should The quality plan.
AS ISO 100052018 pdf download Free Standards Download
Annex b provides a schematic representation of a process approach applied to a quality plan 5 content of the quality plan. Specifically, iso 10005:2018 presents guidelines for establishing, reviewing, accepting, applying, and revising quality plans. It is based on iso 10005 quality management — guidelines for quality plans. Text in blue indicates what information should.
ISO 100052018 Quality management — Guidelines for quality plans
Web this document gives guidelines for establishing, reviewing, accepting, applying and revising quality plans. Annex b provides a schematic representation of a process approach applied to a quality plan These guidelines are not intended to be used as a checklist for compliance with requirements. The quality plan should include or make reference to the plan(s).
Norma Iso 10005
Annex a provides examples of simple quality plans. 4.2 inputs to the quality plan. This document is applicable to quality plans for any intended output, whether a process, product, service, project or contract, and any type or size of organization. Specifically, iso 10005:2018 presents guidelines for establishing, reviewing, accepting, applying, and revising quality plans. 4.1.
Скачать ISO 100052005 Системы менеджмента качества. Руководящие
Annex b provides a schematic representation of a process approach applied to a quality plan Quality plan iso 10005 created date: Provides guidelines to assist suppliers in the preparation, review, acceptance and revision of quality plans. Web clause 5 describes the process of developing a quality plan. Web quality plan iso 10005 keywords: 4 development.
Iso 10005 Quality Plan Template Web the guidance on quality plans in this document is based on the quality management principles described in iso 9000 and the concepts used in iso 9001 for the establishment of quality management systems. Web quality plans prepared under iso 10005:2018 can be used in the context of an established quality management system, such as one created with iso 9001:2015 guidance, or as an independent management activity. Web 4 using a quality plan. 4.2 inputs to the quality plan. The quality plan should include or make reference to the plan(s) for design and development.
These Guidelines Are Not Intended To Be Used As A Checklist For Compliance With Requirements.
A qms quality management plan word template can also help to increase the efficiency of your business. In general, quality plan is not a specific requirements from the iso 13485:2016 or from fda quality system regulation 21 cfr 820; It would have the same requirements as iso 9001 qms but intended for implementation at project level. Web project quality plan (pqp) cpd accredited provider.
Web Clause 5 Describes The Process Of Developing A Quality Plan.
Clause 7 describes the operation and control of a quality plan. Web 4 using a quality plan. Project quality plan (pqp) is normally required for criticality “1” packages. Clause 6 describes the typical contents of a quality plan.
Annex B Provides A Schematic Representation Of A Process Approach Applied To A Quality Plan
Provides guidelines to assist suppliers in the preparation, review, acceptance and revision of quality plans. Quality plan iso 10005 created date: The guide has just been updated to provide more guidance and examples to be relevant to organizations of all shapes and sizes. Clause 6 describes the typical contents of a quality plan.
Iso 10005:2005 Provides Guidelines For The Development, Review, Acceptance, Application And Revision Of Quality Plans.
However it is an essential tool that can help medical device manufacturer to deliver on specific projects. Critical components of quality management plan (iso 10005) quality standards: Web quality plans prepared under iso 10005:2018 can be used in the context of an established quality management system, such as one created with iso 9001:2015 guidance, or as an independent management activity. Web iso 10005 was created in response to the growing demand for quality management systems, and it can be applied to any organization.